Aim of this study is to describe a multicenter experience on percutaneous transhepatic biliary drainage (PTBD) performed with ultrasound-guidance to access the biliary tree, focusing on safety, effectiveness and radiation dose exposure; differences between right- and left-sided approaches have been also evaluated.
Safety and effectiveness of ultrasound-guided percutaneous transhepatic biliary drainage: a multicenter experience
Francesco Giurazza,1 Fabio Corvino,1 Andrea Contegiacomo,2 Paolo Marra,3 Nicola Maria Lucarelli,4 Marco Calandri,5 Mattia Silvestre,1 Antonio Corvino,6 Pierleone Lucatelli,7 Francesco De Cobelli,3 Raffaella Niola,1 Maurizio Cariati,8 and Italian College of Interventional Radiology (ICIR) Rising Stars Group
Author information Article notes Copyright and License information PMC Disclaimer
Abstract
Aims
Aim of this study is to describe a multicenter experience on percutaneous transhepatic biliary drainage (PTBD) performed with ultrasound-guidance to access the biliary tree, focusing on safety, effectiveness and radiation dose exposure; differences between right- and left-sided approaches have been also evaluated.
Methods
This is a multicenter prospective single-arm observational study conducted on patients affected by biliary tree stenosis/occlusion with jaundice and endoscopically inaccessible. The procedures have been performed puncturing the biliary system under US guidance and crossing the stenosis/occlusion under fluoroscopy. Beam-on time and X-ray dose have been evaluated.
Results
117 patients affected by biliary tree stenosis/occlusion not manageable with an endoscopic approach have been included in this analysis. The biliary stenosis/occlusion was malignant in 90.8% and benign in 9.2%. Technical success, considered as positioning of a drainage tube into the biliary tree, was 100%. Overall clinical success, considered as decrease in total bilirubin level after a single procedure, was 95.7%. The overall mean number of liver punctures to catheterize the biliary tree was 1.57. The mean total beam-on time was 570.4 s; the mean dose-area product was 37.25 Gy cm2. No statistical significant differences were observed in terms of technical and dosimetry results according to right-sided and left-sided procedures. Complications rate recorded up to 30 days follow-up was 10.8%, all of minor grades.
Conclusions
In this series US guidance to access the biliary tree for PTBD was a safe and effective technique with an acceptable low-grade complications rate; the reported radiation dose is low.
Graphic abstract
Keywords: Biliary drainage, Percutaneous, Ultrasound, Complications, Radiation dose
Introduction
In the treatment of malignant or benign obstructive jaundice, percutaneous transhepatic biliary drainage (PTBD) represents the gold standard approach in case of endoscopically inaccessible biliary tree [1].
The reported success rate of PTBD is elevated, especially in case of dilated bile ducts allowing an easier percutaneous puncture of the biliary tree [2]. Biliary puncture represents indeed one of the most challenging procedural step and multiple attempts to imply higher bleeding complications rates; therefore, the overall complication rate of percutaneous biliary procedures can be substantial [3–6].
Traditionally biliary puncture has been performed under fluoroscopic guidance using anatomical landmarks [7]; nowadays the procedural technique varies according to operator preference and experience [2, 8].
Another feasible strategy to access the biliary tree is under ultrasound (US) guidance, but very few literature data have been reported and mainly on small cohorts of patients [2, 9–11]; Lee et al. [12] reported their successful experience on a sample of 50 patients with non-dilated bile ducts combining US and fluoroscopic guidance. Ignee et al. [13] described the feasibility of extravascular contrast-enhanced ultrasound on 38 patients to monitor the success of insertion of needle and catheter in PTBD.
The aim of this study is to describe a multicenter experience on PTBD performed with US guidance to access the biliary tree, focusing on safety, effectiveness and radiation dose exposure; differences between right- and left-sided approaches have also been evaluated.
Materials and methods
Sample
This is a multicenter prospective single-arm observational study conducted on patients affected by biliary tree stenosis or occlusion with jaundice endoscopically inaccessible. The endoscopic approach was excluded because of failure of endoscopic catheterization due to lesions localization, gastroduodenal obstructions not susceptible for endoscopic dilatation and postsurgical altered gastrointestinal anatomy.
Written informed consent was obtained from all patients before the procedure.
PTBD performed from January to December 2018 have been included in this analysis.
Data have been collected from electronic medical records and imaging archiving systems in public hospitals and academic interventional radiology departments.
Collected demographic data included: age, gender, bilirubin value, dilation of the biliary tree, stenosis versus occlusion, anatomical level of the biliary stenosis/occlusion, past biliary interventions, etiology of the lesions.
Procedural evaluated factors were as follows: technical and clinical successes, right-/left-sided access, number of liver punctures (intended as passage of the needle through the hepatic capsule) to gain the biliary tree, fluoroscopic time from puncture to stenosis/occlusion level, fluoroscopic time from puncture to end of procedure, radiation dose, short-term (up to 30 days) complication rates. Differences in the mentioned values between procedures with right and left lobe access have been evaluated too.
Procedural protocol
All the operators work in Interventional Radiology departments of tertiary care level centers and have 4–10 years of experience in percutaneous biliary interventions.
The procedures have been performed puncturing the biliary system under US guidance and then crossing the stenosis/occlusion under fluoroscopic guidance. In all cases, the operators attempted to position an internal biliary drainage; if the occlusion was uncrossable, an external drainage was fixed above the occlusion.
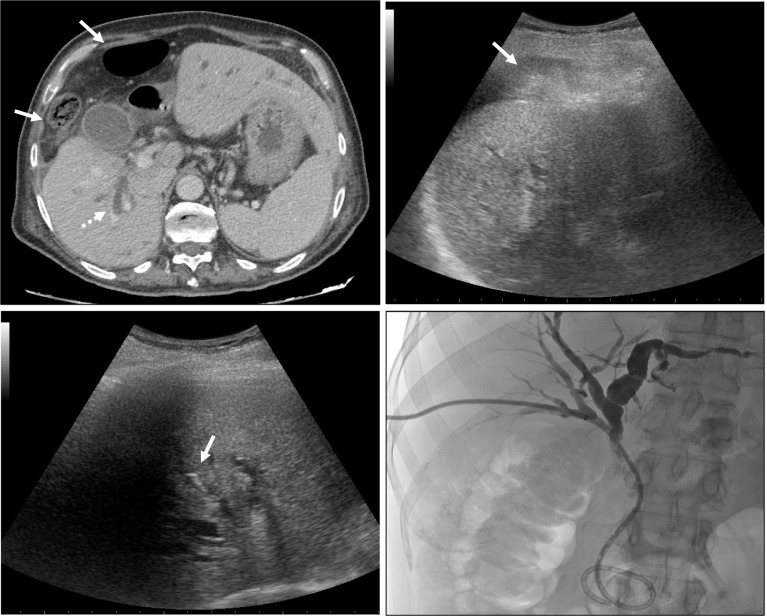
The interventional strategy has always been planned on the basis of preprocedural imaging findings, including Computed Tomography (CT) and/or Magnetic Resonance (MR) examinations; in details, evaluation of stenosis/occlusion level and interposing anatomical limiting factors (bowels, and ascites) were evaluated to assess the best access to biliary system (Figs. 1, ,22a).

Fig. 1
a, b 63 year old man with jaundice due to hilar occlusion by a cholangiocarcinoma; venous contrast-enhanced CT scan in axial plane showing colon loops (white arrows) transposition between liver and abdominal wall, Chilaiditi syndrome. In a and in b is evident right and left lobe biliary ducts dilation (white dotted arrows), respectively. c 58 years old woman with pancreas cancer and jaundice due to distal occlusion of the choledocus causing intrahepatic biliary ducts dilation (dotted white arrows); perihepatic ascites is evident (white arrow)

Fig. 2
Same patient of Fig. 1a, b. Intercostal right lobe ultrasound scan: a colon loops interposition (white arrow) limits the access to the right-sided biliary tree. b A proper intercostal access window is individuated to avoid bowel loops and a 22-gauge spinal needle (dotted white arrow) is positioned up to the liver capsule to inject local anesthetic. c A 21 gauge Chiba needle tip (black arrow) is conducted up to the selected biliary branch. The same biliary puncture technique was adopted also for left lobe; these patients received bilateral internal PTBD due to hilar occlusion
Laboratory liver (transaminases, bilirubin, alkaline phosphatase, and gamma glutamyl transferase) and renal (creatinine) function tests as well as coagulation status were routinely monitored; according to the SIR-CIRSE guidelines for significant bleeding risk procedures [14], transfusions were performed in case of platelets value < 50,000 and indexed normalized ratio (INR) was corrected if > 1.5. Patients assuming clopidogrel and aspirin were strictly recommended to withhold for 5 days before the procedure.
Wide-spectrum antibiotics and antiemetics were administered prophylactically in accordance with specific-site protocols. Moderate sedation with fentanyl and/or midazolam was generally performed for pain relief; the procedures requiring deep sedation with propofol were managed in synergy with anesthesiologists.
Based on local facilities, the ultrasounds adopted in the angio-suite were Esaote® MyLab25—MyLab70—MyLab ClassC—MyLab Twice, Toshiba® Aplio500 and Philips® iU22-Affiniti 70G.
First, local anesthetic was injected (max. 10–15 ml of xylocaine/carbocaine) using a 22 gauge spinal needle positioned subcutaneously and up to the liver capsule under US guidance corresponding to the selected point of entrance into the liver parenchyma (Fig. 2b).
Right and left lobes were punctured, respectively, with intercostal and subcostal epigastric accesses to reduce the risk of bleeding and guarantee stability of the drainage catheter.
The liver puncture was performed using micropuncture kits (Neff Percutaneous Access Set, CookMedical®—AccuStick Introducer System, Boston Scientific®). The biliary system was approached as peripheral as possible and avoiding ducts bifurcations to reduce the risks of bleeding and incomplete drainage.
A 21 gauge Chiba needle was conducted under US guidance with 3.5-MHz convex probe up to the selected biliary branch (Fig. 2c), a microguidewire was then advanced as close as possible to the stenosis/occlusion under US and fluoroscopic guidance, finally a working cannula was positioned. Using a hydrophilic guidewire and a 4Fr/5Fr diagnostic catheter, with different tips morphology according to the anatomical findings, the stenosis/occlusion was crossed reaching the duodenum or the efferent jejunal loop in case of bilio-enteric anastomoses. Bilioplasty with balloon catheter was performed whenever required to treat benign strictures or stent occlusion (Fig. 3). Finally, on a stiff guidewire an internal drainage was positioned and fixed to the skin with sutures and patches. In case of large amount of ascites which may increase the risk of bleeding and drainage dislodgement, a 5Fr pig-tail catheter was positioned in the perihepatic space (Figs. 3, ,4)4) to drain the liquids. The PTBD drainage calipers were 8Fr/8.5Fr/10Fr according to operators’ preference and availability. In case of uncrossable occlusion, an external drainage was positioned and after 48–72 h a second procedural session was scheduled to attempt crossing the lesion with reduced biliary tree dilation.

Fig. 3
Same patient of Fig. 1c; this patient was treated 4 months before with percutaneous metallic stenting of the distal choledocus which was occluded by adenocarcinoma of pancreas head. a Venous enhanced CT scan reconstruction in coronal plane showing perihepatic ascites (black asterisk), choledocus dilation (white dotted arrow) and the occluded metallic stent (black arrow). b At the beginning of the procedure, a 5Fr pig tail catheter (white arrow) was positioned in the perihepatic space to drain the ascites; the metallic stent is evident (black arrow); this patient had also previous cardio-thoracic surgery and so metallic juncture of the sternum is evident. c After recanalization of the metallic stent (black arrow), a bilioplasty was performed; radiopaque markers of the balloon are indicated by grey arrows. d A 8.5Fr intercostal internal PTBD was positioned; small amount of hemobilia (black dotted arrow) was caused by the bilioplasty without disfunction of the drainage neither clinical effects. Extrahepatic contrast extravasation in correspondence of the point of entrance of the PTBD is also evident (black circle); this finding is common in case of patients with ascites and in small amount does not produce technical neither clinical consequences

Fig. 4
66 year old woman with duodenal carcinoma infiltrating the liver hilum. a Perihepatic ascites (white arrow) and right lobe biliary ducts dilation (white dotted arrow) are evident. b As for the patient in Fig. 3, a 5Fr pig tail catheter was positioned in the perihepatic space to drain ascites (black arrow); with the same technique described in Fig. 2, the biliary duct of the VI segment was punctured with a 21 gauge Chiba needle (black dotted arrow) with subsequent opacification of the biliary tree (grey arrow). c a 8.5Fr intercostal internal PTBD was positioned
The X-ray dose was reduced as much as possible using as low as reasonably achievable (ALARA) principles, especially minimizing X-ray preferring fluoroscopy, applying beam collimation and increasing the distance from patient to beam source.
Statistical analysis
All the statistics were developed in the MATLAB® (MathWorks, Inc., Natick, Massachusetts, USA) environment.
Comparison between procedural data of interventions performed with right lobe and left lobe accesses was evaluated: differences were considered statistically significant if p value < 0.05, using Student’s t test or Wilcoxon signed-rank test.
Results
Demographic data
117 patients affected by biliary tree stenosis/occlusion not manageable with an endoscopic approach have been included in this analysis; three patients required bilateral PTBD, therefore the overall number of biliary drainages positioned was 120.
Descriptive population data are reported in Table 1.
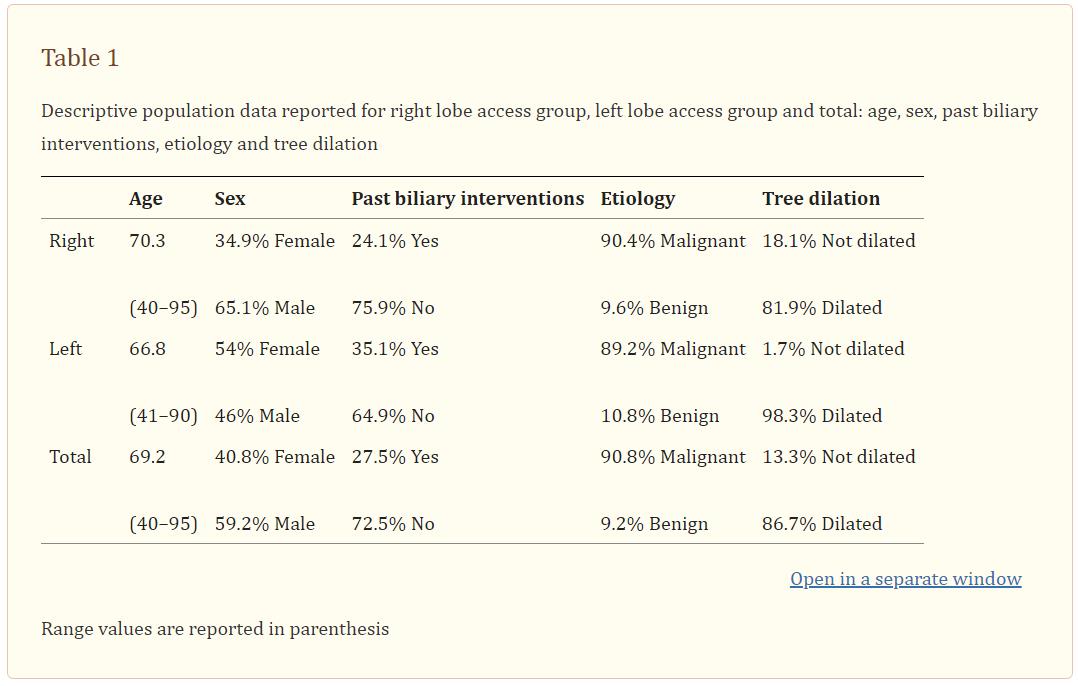
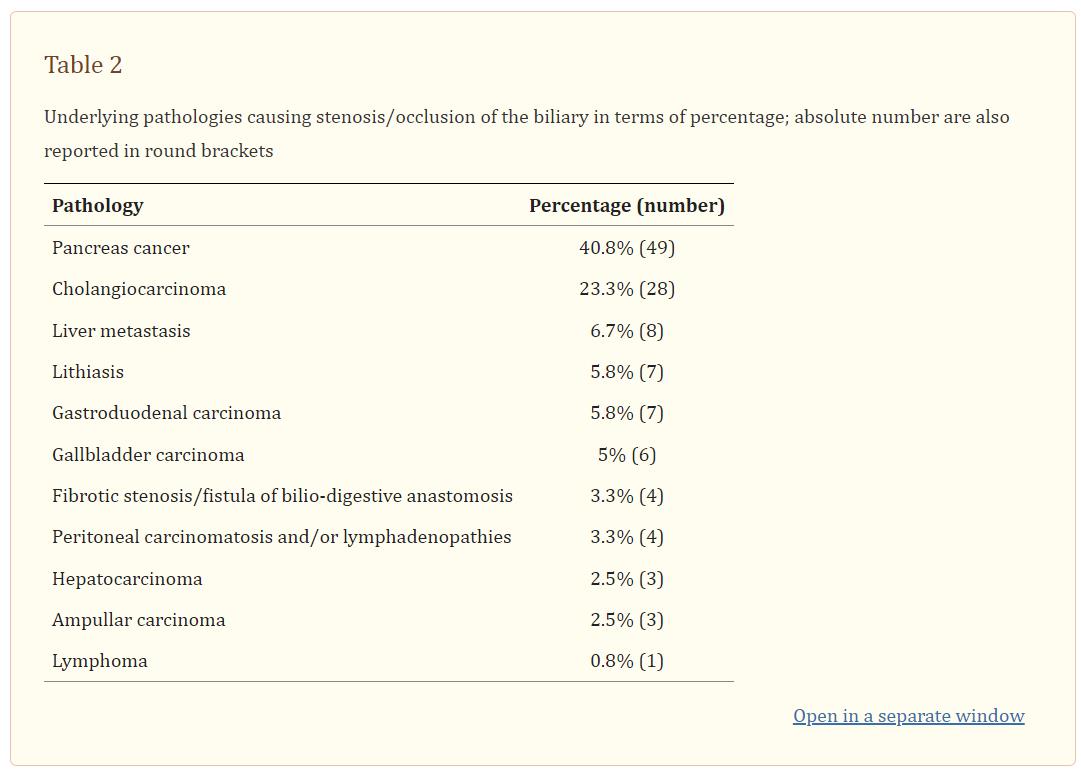
Mean age was 69.2 years (range: 40–95), 40.8% females and 59.2% males. The mean total bilirubin level was 14.1 mg/dl (range 1.9–43.8). In 27.5% of patients’ previous interventions involving the biliary tree were recorded [endoscopic retrograde cholangiopancreatography (ERCP), percutaneous biliary drainage, biliary stenting, and intraluminal biliary biopsies]. The biliary stenosis/occlusion was malignant in 90.8% of patients while it was benign in 9.2% (Table 2). Pancreas cancer and lithiasis were the most common pathologies among malignant and benign pathologies, respectively.
The intrahepatic biliary tree appeared dilated at preprocedural US in 86.7%.
Procedural data
Procedural data are summarized in Table 3.

Range values are reported in parenthesis. Standard deviation values are reported in parenthesis Standard deviation values are reported in parenthesis No. number, s seconds, Gy gray, cm centimeter
Procedural cholangiography showed in 22.5% a stenosis and in 77.5% an occlusion of the biliary tree; lesions were localized in 71.7% in the common bile duct/choledocus, in 13.3% in the intrahepatic biliary tree, in 10.8% in correspondence of the biliary hilum and in 4.2% in a bilio-enteric anastomosis.
The technical success, considered as positioning of a drainage tube into the biliary tree, was 100%.
In 88.3% of cases an internal drainage was positioned while in 11.7% the occlusion was uncrossable at first attempt and so an external drainage was left in place. According to the local preference and availability, the caliper of the drainage was 8Fr in 51.7%, 8.5Fr in 25.8% and 10Fr in 22.5%.
The overall clinical success, considered as decrease in total bilirubin level after a single procedure, was 95.7%.
In 69.2% of cases a right-sided intercostal approach was adopted while in 30.8% a subcostal epigastric left-sided access was chosen; in case of intrahepatic lesions, the site access depended on the lobe involved; in case of hilar disease operators punctured the lobe presenting the larger bile duct dilation; in case of common bile duct/choledocus occlusion, the site access depended on operator preference.
The overall mean number of liver puncture performed to catheterize the biliary tree was 1.57 (1.67 for left lobe and 1.52 for right lobe, range: 1–8).
The mean total beam-on time was 570.4 s (range: 59–3060) while the mean beam-on time occurring to reach the biliary stenosis/occlusion was 146.8 s (range: 3–1080). Concerning the X-ray dose assessment, the dose-area product (DAP) reported by the angiography machine was considered: in mean it was 37.25 Gy cm2 (range: 1.16–212.43).
Subdividing the sample in two subgroups according to right- or left-sided access, no statistical significant differences were observed in terms of: internal or external drain, number of punctures, total beam-on time, beam-on time from liver puncture to biliary stenosis/occlusion, radiation dose expressed as DAP, complications rate (Table 3).
Complications
Complications were evaluated according to the CIRSE Standards for classification of complications [15].
Complications rate recorded up to 30 days follow-up was 10.8% (13 patients). Two cases (1.7%) of minor bleeding coming out from the drainage and due to a peripheral portal branch lesion were reported: one self-limited without clinical sequelae (grade I); the other required, 2 days after the procedure, drainage tube replacement because it was occluded by clots (grade III) and so entailing a prolonged hospital recovery. Drainage displacement requiring new hospital access occurred in 5% of the patients (6) up to 1 week after positioning (grade III). Fever due to cholangitis requiring medical therapy presented in 3.3% (4) (grade III). Finally in one case (0.8%) a small (< 2 cm) subcapsular biloma was detected at follow-up CT, not requiring specific treatment because of self-limiting with patient asymptomatic (grade I).
Discussion
From the first description of fluoro-guided PTBD in 1962 [16] several techniques have been proposed without clear standardization. According to literature data, procedural related complications rates are substantial even if heterogeneous [2]. Especially for biliary ducts puncture, multiple fluoroscopic and US-guided approaches have been reported. Therefore, it is relevant to adopt an effective strategy because multiple unsuccessful liver punctures can lead to major complications related to bleeding and higher radiation doses.
Even if US-guided PTBD has been described as an effective and safe method since time [10], only limited data are available in literature [2, 9–12, 17, 18]. Actually, both fluoro-guided and US-guided techniques are adopted in the clinical practice and the choice depends mainly on operator preference; some interventionalists prefer US guidance only for technically challenging cases and left-sided puncture, to avoid gastrointestinal structures [19, 20]. Moreover, US guidance may allow to avoid the accidental puncture of liver lesions in case of bilobar intrahepatic metastatic disease.
The SIR guidelines for PTBD recommended a procedural threshold for major complications of 10% and a biliary tree cannulation success rates of 95% and 70% in case of dilated and non-dilated ducts, respectively [1]. In this series the complication rate was 10.8%, with low grades (all between grade I and grade III of CIRSE classification complications system), while the cannulation of the biliary tree was feasible in 100%; these data, compared to literature-reported outcomes of fluoro-guided PTBD [1–6, 12, 16, 19, 20], seems to encourage US guidance. This is in accordance with two previous published studies in 1995 [17] and 2004 [9] and a recent study of 2017 [6] which described an advantageous overall interventional complications rate for US-guided PTBD over fluoro-guidance.
On the other hand, Nennstiel et al. [2] recently reported their experience on a sample of 195 patients comparing fluoroscopic versus US-guided approach in terms of success and complications rates; they performed 207 fluoro-guided and 44 US-guided PTBD. They concluded that the fluoroscopic approach is superior for draining the right biliary system, even if entailing major complications compared to the US guidance. Concerning radiation dose evaluation, they reported only the fluoroscopic time but no data about the radiation dose.
Compared with literature data [2, 6, 21], the fluoroscopic time in this series seems to be low; this is probably due to the avoidance of beam-on time to access the biliary tree. Furthermore, according to a report published in 2017 [22] by the Italian Healthcare Committee (ISS) about the radiological dosimetry levels for interventional radiology procedures, the threshold DAP value for biliary intervention should be 43 Gy cm2. Few papers have analyzed the radiation dosimetry during biliary interventions: among these, Kloeckner et al. [21] reported a mean DAP value of 129.8 Gy cm2 and 259.4 Gy cm2 for right and left PTBD, respectively, performed by senior radiologists. Actually the mean DAP value reported in this series is lower (37.25 Gy cm2). To reduce procedural time and consequently the radiation dose, some authors [23] have recently proposed an intraoperative referencing system using a virtual fluoroscopic preprocedural planning to better localize the guidewire in the bile duct.
Comparing right- and left-sided biliary procedures in terms of procedural data and complications rate, in this study no significant differences were appreciated (Table 3). Houghton et al. [19] reported on a sample of 150 patients a mean number of 3 liver punctures for right lobe access and 2 for left lobe, with a low significant bleeding complications rate (0.66%) even if higher for right lobe access; on the other hand, two independent groups of authors reported higher risk of arterial bleeding for left lobe access procedures [24, 25]. This is coherent with anatomical knowledge: in the left lobe arterial and portal branches run ventrally to the biliary tree and may be intercepted along the PTBD track.
Compared to literature data [26], the low rate of cholangitis-related fever may be due to the high percentage of internal drainage positioned.
An alternative to PTBD may be endoscopic ultrasound-guided biliary drainage (EGBD): this novel procedure involves accessing the biliary tree from within the lumen of the gastrointestinal system using echoendoscopy and fluoroscopy, creating a fistulous tract and deploying a stent in a single-step procedure, obviating the need for external drain [27]. Compared to PTBD, main advantages of this procedure are a better nutrition absorption avoiding electrolyte loss and preventing the stress of external drain [28]; however, the procedure is technically complex requiring specialized training and a steep learning curve to avoid complications as bile leak, pneumoperitoneum, bleeding and stent migration. At moment this approach still lacks sufficient evidence to be applied on a large scale and prospective robust trials are necessary [29].
Today, thanks to technological progresses, US imaging quality has reached a high standard level allowing long-distance visualization of the biliary tree. This imaging modality is operator-dependent and is widely adopted by interventional radiologists for multiple percutaneous procedures and diagnostic exams [30]. Superiority in terms of safety and effectiveness of US guidance over free hand and fluoro-guidance has already been clarified for central vascular accesses [31–33]. Furthermore it is empirically noted that modern generations of interventionalists, as the operators of this manuscript, handle the US guidance familiarly [34, 35]; so, the use of this technique to perform percutaneous access of the biliary tree is probably intended to increase.
This study presents limitations. First of all, there is no direct comparison with a fluoro-guided PTBD group by the same operators; this is because the authors routinely perform biliary punctures with US guidance. The largest part of the procedures has been conducted with right lobe access (69.2%) and on malignancies (90.8%), so conclusions may be limited concerning PTBD with left lobe access and on patients with benign pathologies; however, it should be noted that in the routinary clinical practice the largest part of PTBD is executed on neoplastic patients; also, until today, there is no clear superiority of right- versus left-sided access, so when feasible the choice depends on operator preference. The follow-up data are limited by short term, 30 days after the procedure, but it should be considered that these malignant patients present usually short life expectancy. Finally, even if based on authors’ knowledge, this is one of the largest reported sample on US-guided biliary PTBD, further wide cohorts trials are required to support the described outcomes.
In conclusion, in this series, US guidance to access the biliary tree for PTBD was a safe and effective technique with an acceptable complications rate and of low grade; considering the existing literature data, the reported radiation dose is low. No significant differences in terms of procedural outcomes have been noted between right and left lobe approaches.
Go to:
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent
Informed consent was obtained from all individual patients included in the study.
Human and animal and rights
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Go to:
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Go to:
Contributor Information
Francesco Giurazza, Email: ti.liamtoh@azzaruigocsecnarf.
Fabio Corvino, Email: moc.liamg@onivroceffe.
Andrea Contegiacomo, Email: moc.liamg@omocaigetnoc.aerdna.
Paolo Marra, Email: ti.rsh@oloap.arram.
Nicola Maria Lucarelli, Email: moc.liamg@ocin.illeracul.
Marco Calandri, Email: ti.evil@irdnalac.ocram.
Mattia Silvestre, Email: moc.liamg@dmertsevlisaittam.
Antonio Corvino, Email: ti.liamtoh@roc.na.
Pierleone Lucatelli, Email: moc.liamg@illetacul.enoelreip.
Francesco De Cobelli, Email: ti.rsh@ocsecnarf.illeboced.
Raffaella Niola, Email: moc.liamg@2aloinalleaffar.
Maurizio Cariati, Email: ti.olracoloapitnas-tssa@itairac.oiziruam.
Go to:
References
1. Saad WE, Wallace MJ, Wojak JC, Kundu S, Cardella JF. Quality improvement guidelines for percutaneous transhepatic cholangiography, biliary drainage, and percutaneous cholecystostomy. J Vasc Interv Radiol. 2010;21(6):789–795. doi: 10.1016/j.jvir.2010.01.012. [PubMed] [CrossRef] [Google Scholar]
2. Nennstiel S, Treiber M, Faber A, et al. Comparison of ultrasound and fluoroscopically guided percutaneous transhepatic biliary drainage. Dig Dis. 2019;37(1):77–86. doi: 10.1159/000493120. [PubMed] [CrossRef] [Google Scholar]
3. Kühn JP, Busemann A, Lerch MM, Heidecke CD, Hosten N, Puls R. Percutaneous biliary drainage in patients with nondilated intrahepatic bile ducts compared with patients with dilated intrahepatic bile ducts. AJR Am J Roentgenol. 2010;195:851–857. doi: 10.2214/AJR.09.3461. [PubMed] [CrossRef] [Google Scholar]
4. Oh HC, Lee SK, Lee TY, Kwon S, Lee SS, SeoDW Kim MH. Analysis of percutaneous transhepatic cholangioscopy-related complications and the risk factors for those complications. Endoscopy. 2007;39:731–736. doi: 10.1055/s-2007-966577. [PubMed] [CrossRef] [Google Scholar]
5. Weber A, Gaa J, Rosca B, et al. Complications of percutaneous transhepatic biliary drainage in patients with dilated and nondilated intrahepaticbile ducts. Eur J Radiol. 2009;72:412–417. doi: 10.1016/j.ejrad.2008.08.012. [PubMed] [CrossRef] [Google Scholar]
6. Wagner A, Mayr C, Kiesslich T, Berr F, Friesenbichler P, Wolkersdörfer GW. Reduced complication rates of percutaneous transhepatic biliary drainage with ultrasound guidance. J Clin Ultrasound. 2017;45:400–407. doi: 10.1002/jcu.22461. [PubMed] [CrossRef] [Google Scholar]
7. Kandarpa K, Aruny JE. Handbook of interventional radiologic procedures. 3. Philadelphia: Lippincott Williams & Wilkins; 2002. [Google Scholar]
8. Dumonceau JM, Tringali A, Blero D, et al. European Society of Gastrointestinal Endoscopy: Biliary stenting: indications, choice of stents and results: European Society of Gastrointestinal Endoscopy (ESGE) clinical guideline. Endoscopy. 2012;44:277–298. doi: 10.1055/s-0031-1291633. [PubMed] [CrossRef] [Google Scholar]
9. Kozlov AV, Polikarpov AA, Oleshchuk NV, Tarazov PG. Comparative assessment of percutaneous transhepatic cholangio drainage under roentgenoscopy and US guidance. Vestn Rentgenol Radiol. 2002;4:30–33. [PubMed] [Google Scholar]
10. Makuuchi M, Bandai Y, Ito T, et al. Ultrasonically guided percutaneous transhepatic bile drainage: a single-step procedure without cholangiography. Radiology. 1980;136:165–169. doi: 10.1148/radiology.136.1.7384494. [PubMed] [CrossRef] [Google Scholar]
11. Sukigara M, Taguchi Y, Watanabe T, Koshizuka S, Koyama I, Omoto R. Percutaneous transhepatic biliary drainage guided by color Doppler echography. Abdom Imaging. 1994;19:147–149. doi: 10.1007/BF00203490. [PubMed] [CrossRef] [Google Scholar]
12. Lee W, Kim GC, Kim JY, et al. Ultrasound and fluoroscopy guided percutaneous transhepatic biliary drainage in patients with nondilated bile ducts. Abdom Imaging. 2008;33:555–559. doi: 10.1007/s00261-007-9349-3. [PubMed] [CrossRef] [Google Scholar]
13. Ignee A, Cui X, Schuessler G, Dietrich CF. Percutaneous transhepatic cholangiography and drainage using extravascular contrast enhanced ultrasound. Z Gastroenterol. 2015;53(5):385–390. doi: 10.1055/s-0034-1398796. [PubMed] [CrossRef] [Google Scholar]
14. Patel IJ, Davidson JC, Nikolic B, et al. Consensus guidelines for periprocedural management of coagulation status and hemostasis risk in percutaneous image-guided interventions. J Vasc Interv Radiol. 2012;23(6):727–736. doi: 10.1016/j.jvir.2012.02.012. [PubMed] [CrossRef] [Google Scholar]
15. Filippiadis DK, Binkert C, Pellerin O, Hoffman RT, Krajina A, Pereira PL. Cirse quality assurance document and standards for classification of complications: the cirse classification system. Cardiovasc Intervent Radiol. 2017;40:1141–1146. doi: 10.1007/s00270-017-1703-4. [PubMed] [CrossRef] [Google Scholar]
16. Glenn F, Evans JA, Mujahed Z, Thorbjarnarson B. Percutaneous transhepatic cholangiography. Ann Surg. 1962;156:451–462. doi: 10.1097/00000658-196209000-00012. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
17. Takada T, Yasuda H, Hanyu F. Technique and management of percutaneous transhepatic cholangial drainage for treating an obstructive jaundice. Hepatogastroenterology. 1995;42:317–322. [PubMed] [Google Scholar]
18. Righi D, Doriguzzi A, Rampado O, et al. Interventional procedures for biliary drainage with bilioplasty in paediatric patients: dosimetric aspects. Radiol Med. 2008;113:429–438. doi: 10.1007/s11547-008-0252-y. [PubMed] [CrossRef] [Google Scholar]
19. Houghton EJ, Zeledon M, Acquafresca P, Finger C, Palermo M, Gimenez ME. Prospective comparison of bleeding complications between right and left approaches in percutaneous biliary drainage. Surg Laparosc Endosc Percutan Tech. 2018 doi: 10.1097/sle.0000000000000609. [PubMed] [CrossRef] [Google Scholar]
20. Corvino F, Centore L, Soreca E, Corvino A, Farbo V, Bencivenga A. Percutaneous “Y” biliary stent placement in palliative treatment of type 4 malignant hilar stricture. J Gastrointest Oncol. 2016;7(2):255–261. [PMC free article] [PubMed] [Google Scholar]
21. Kloeckner R, Bersch A, Pinto dos Santos D, Schneider J, Duber C, Pitton MC. Radiation Exposure in nonvascular fluoroscopy-guided interventional procedures. Cardiovasc Intervent Radiol. 2012;35:613–620. doi: 10.1007/s00270-011-0206-y. [PubMed] [CrossRef] [Google Scholar]
22. Padovani R, Compagnone G, D’Ercole L, Orlacchio A, Bernardi G, Rosi A, Campanella F (2017) Livelli diagnostici di riferimento nazionali per la radiologia diagnostica e interventistica. Roma: Istituto Superiore di Sanità (Rapporti ISTISAN 17/33)
23. Kinoshita M, Shirono R, Takechi K, et al. The usefulness of virtual fluoroscopic preprocedural planning during percutaneous transhepatic biliary drainage. Cardiovasc Intervent Radiol. 2017;40:894–901. doi: 10.1007/s00270-017-1581-9. [PubMed] [CrossRef] [Google Scholar]
24. Choi SH, Gwon DI, Ko GY, et al. Hepatic arterial injuries in 3110 patients following percutaneous biliary drainage. Radiology. 2011;261:969–975. doi: 10.1148/radiol.11110254. [PubMed] [CrossRef] [Google Scholar]
25. Rivera-Sanfeliz GM, Assar OS, LaBerge JM, et al. Incidence of important hemobilia following transhepatic biliary drainage left-sided versus right-sided approaches. Cardiovasc Interv Radiol. 2004;27:137–139. doi: 10.1007/s00270-003-0022-0. [PubMed] [CrossRef] [Google Scholar]
26. Lucatelli P, Corradini SG, Corona M, et al. Risk factors for immediate and delayed-onset fever after pecutaneous transhepatic biliary drainage. Cardiovasc Interv Radiol. 2016;39(5):746–755. doi: 10.1007/s00270-015-1242-9. [PubMed] [CrossRef] [Google Scholar]
27. Baniya R, Upadhaya S, Madala S, Subedi SC, Mohammed TS, Bachuwa G. Endoscopic ultrasound-guided biliary drainage versus percutaneous transhepatic biliary drainage after failed endoscopic retrograde cholangiopancreatography: a meta-analysis. Clin Exp Gastroenterol. 2017;10:67–74. doi: 10.2147/CEG.S132004. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
28. Holt BA, Hawes R, Hasan M, et al. Biliary drainage: role of EUS guidance. Gastrointest Endosc. 2016;83(1):160–165. doi: 10.1016/j.gie.2015.06.019. [PubMed] [CrossRef] [Google Scholar]
29. De Cobelli F, Marra P, Diana P, Brembilla G, Venturini M. Therapeutic EUS: Biliary drainage—the interventional radiologist’s perspective. Endosc Ultrasound. 2017;6(Suppl 3):S127–S131. doi: 10.4103/eus.eus_77_17. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
30. Vidili G, De Sio I, D’Onofrio M, et al. SIUMB guidelines and recommendations for the correct use of ultrasound in the management of patients with focal liver disease. J Ultrasound. 2019;22(1):41–51. doi: 10.1007/s40477-018-0343-0. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
31. Jenssen C, Brkljacic B, Hocke M, et al. EFSUMB guidelines on interventional ultrasound (INVUS), Part VI—ultrasound-guided vascular interventions. Ultraschall Med. 2015 doi: 10.1055/s-0035-1553450. [PubMed] [CrossRef] [Google Scholar]
32. Lamperti M, Bodenham AR, Pittiruti M, et al. International evidence-based recommendations on ultrasound-guidedvascular access. Intensive Care Med. 2012;38:1105–1117. doi: 10.1007/s00134-012-2597-x. [PubMed] [CrossRef] [Google Scholar]
33. Tuna Katircibaşi M, Güneş H, Çağri Aykan A, Aksu E, Özgül S. Comparison of ultrasound guidance and conventional method for common femoral artery cannulation: a prospective study of 939 patients. Acta Cardiol Sin. 2018;34(5):394–398. [PMC free article] [PubMed] [Google Scholar]
34. Corvino A, Sandomenico F, Setola SV, Corvino F, Tafuri D, Catalano D. Morphological and dynamic evaluation of complex cystic focal liver lesions by contrast-enhanced ultrasound: current state of the art. J Ultrasound. 2019;1:3. doi: 10.1007/s40477-019-00385-2. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
35. Corvino A, Catalano O, Corvino F, Sandomenico F, Petrillo A. Diagnostic performance and confidence of contrast-enhanced ultrasound in the differential diagnosis of cystic and cystic-like liver lesions. AJR Am J Roentgenol. 2017;209(3):W119–W127. doi: 10.2214/AJR.16.17062. [PubMed] [CrossRef] [Google Scholar]
Articles from Journal of Ultrasound are provided here courtesy of Springer


引用/广播